
Purity transformed into beauty
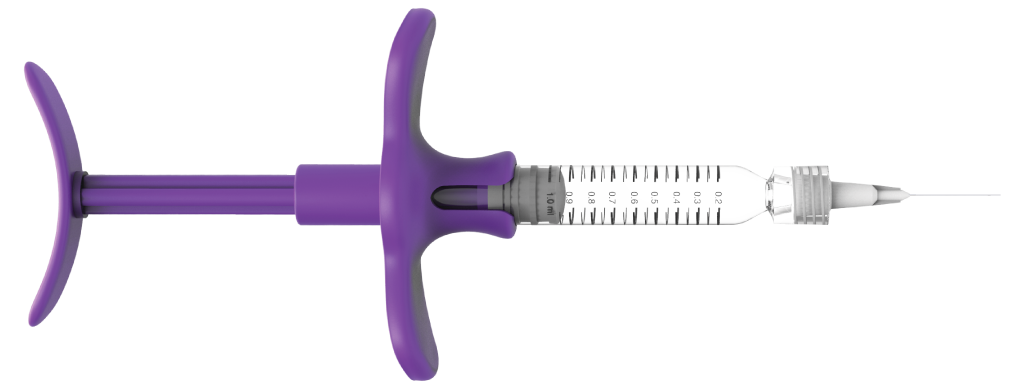
Hyaluronic Acid Dermal Filler
– Temporary improvement of facial wrinkles
– Use of high-purity medical hyaluronic acid raw materials
– Contains local anesthetic lidocaine to improve procedure pain and discomfort
– Contains local anesthetic lidocaine
– Temporarily improves wrinkles with cross-linked hyaluronic acid particles
– Uses pharmaceutical grade hyaluronic acid

MONA LISA
High Purity Hyaluronic Acid
GENOSS implements a strict quality control system through direct participation in the entire production process, from the base material of hyaluronic acid to its final production.
NEW


Long-Term Stability
Better retention
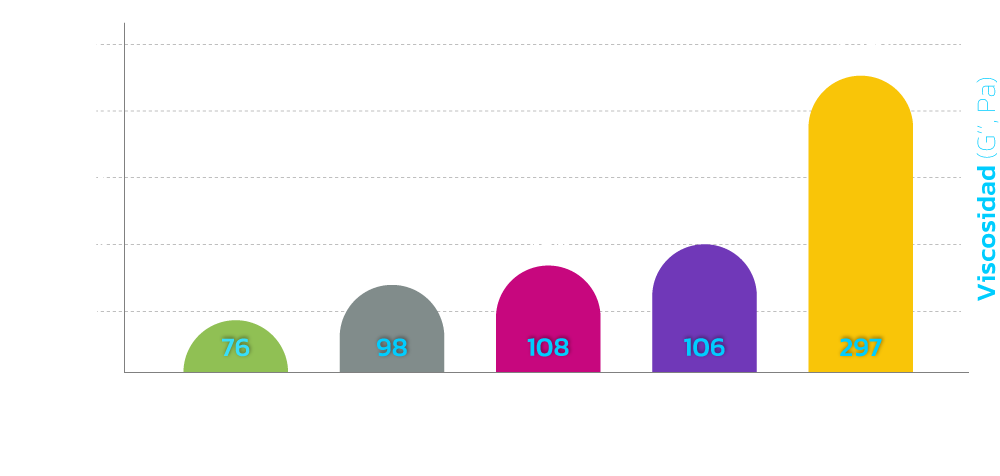
Safety is guaranteed with a lower degree of cross-linking than other products, and excellent stability was confirmed through a hyaluronidase degradation test.

WHY MONALISA
The uniform sized hyaluronic acid particles with optimal
viscoelasticity can maintain a long-lasting volume
MONALISA is safe to use on patients due to low level
of endotoxin and essentially no BDDE residue
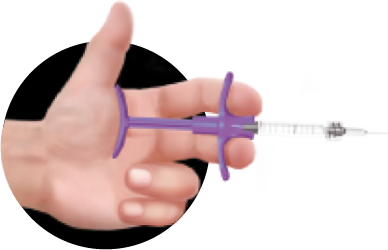
The ergonomically-designed rod and grip allow the even distribution of pressure during injection to enable an accurate and safe treatment for both the clinician and the patient
GENOSS implements a strict quality control system through direct involvement
in the entire production process from the base material of hyaluronic acid to the final product
To guarantee the quality of MONALISA, GENOSS strictly fulfills and complies
with the international quality regulations, including KGMP, ISO 13485 and ISO 9001
APPLICATIONS OF DERMAL FILLER
Fill:
- Smoothes superficial lines such as perioculars and fine lines.
- Reduces moderate lines such as frown lines.
- Reduces moderate wrinkles such as those on the forehead and perioral area.
- Fills deep wrinkles such as the nasolabial fold.
Gives volume:
- For a subtle change like lip definition.
- For an intense change like rhinomodeling.
- For a dramatic change such as mandibular contouring.

Characteristics
Ergonomic design
Greater precision for a comfortable and safe application.


Comfortable grip

Comfortable grip

Anti-slip
Grip
Ergonomic design that fits perfectly in the hands of the specialist, facilitating handling for application to various parts of the face.

Competence
Mona Lisa

Mona Lisa
Thin Wall Needle
Thin-walled needle for smooth patient ejection.
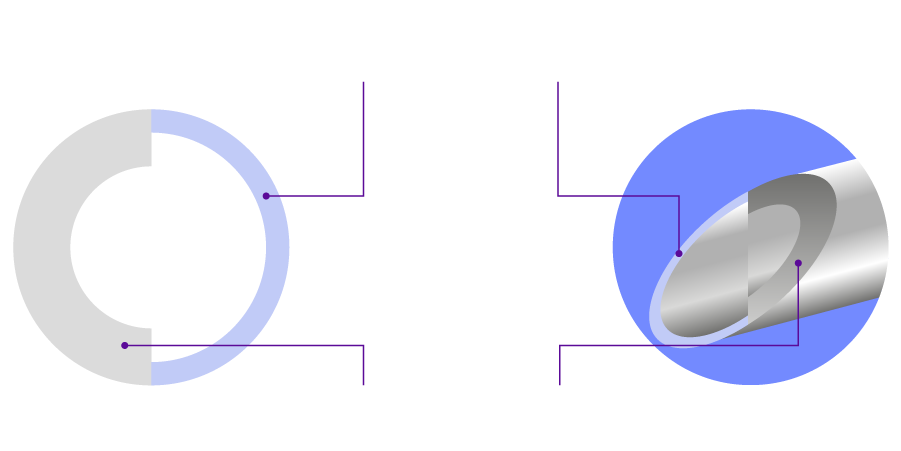

Hyaluronic Acid
Cross-Links
Cross-linking Technology with EP grade Raw Material
- Highly pure Hyaluronic Acid
- Manufacturing Process Complied with ISO 13485, ISO 9001
- Hy-BRID Technology enables MONALISA/MONALISA Lidocaine to have optimal viscoelasticity & long-lasting volume
Hy-BRID Technology
- Hyper Cross-linked
- Based on non-animal HA
- Residual free
- Improved Density and elasticity
Versions and applications
We have an option for every application area! Each version of MONALISA has the specific amount of particles to achieve the result your patients want.
SOFT
ELASTIC

200μm
Crow's feet.
Lower eyelids
Fine lines on the forehead.
Superficial periocular and perioral lines.
MILD
ELASTIC

400μm
Glabellar wrinkles.
Grooves in temporal area.
Deep periocular and perioral lines.
Lip augmentation.
Cheeks.
HARD
ELASTIC

600μm
Forehead.
Chin.
Nose bridge.
Nasolabial groove.
Intense lip growth and definition.
ULTRA
ELASTIC

900μm
Forehead.
Nose bridge.
Nasal augmentation.
Deep nasolabial fold.
cheekbones.
Jaw.
Chin.
HIGH
ELASTIC

900μm
Chin augmentation.
Jaw volume.
Nose bridge
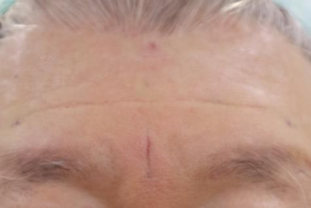
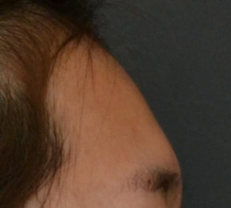
BEFORE AND AFTER
With MONALISA you will obtain the best results in a shorter time and a much longer lasting effect than others.
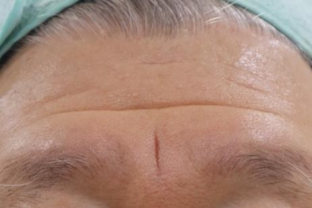
Nasolabial fold Observations:
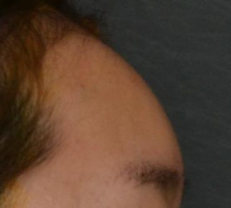
Nasolabial fold Observations:
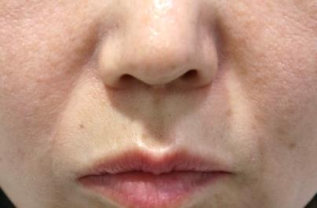
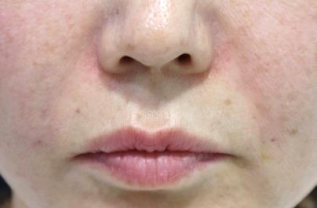
Nasolabial fold Observations:
Safety
MONALISA has lowered the risk of side effects through a strict quality control system.
division | standard | result |
|---|---|---|
shape | No impurities, transparent and colorless gel | Pass |
Hyaluronic acid concentration | 21.6 ~ 26.4mg/mL | 24.1 mg/mL
|
pH | 6.5 ~ 7.5 | 7.10
|
BDDE residual amount | <2ppm | Not Detected/td>
|
Endotoxin | < 0.5 EU/mL | < 0.1 EU/mL
|
Volume | > 1.0mL | > 1.0mL |
* What is BDDE (1,4-butanediol diglycidyl ether, cross-linking agent)?
model name | Internal particle size | Standard capacity | Lidocaine concentration |
|---|---|---|---|
MPF10S | 200㎛ | 1.0ml | 0.3%
|
MPF10M | 400㎛
| ||
MPF10H | 600㎛
| ||
MPF10U | 900㎛
|

